Atomic relative calculate isotopes abundance isotope isotopic spectrometry periodic The periodic table of elements scaled to show the elements' actual Solved: most elements occur naturally as a mix of differen...
Abundance - in a chemical reaction - Assignment Point
Abundance percent chemistry finding Isotopes abundance equation atomic chemistry isotopic fractional mass masses weighted chem libretexts divided Solving for percent relative abundance
Calculating percentage and relative abundance from ar aqa 7404 as topic
How to calculate atomic mass of isotopes archivesAbundance s05 percent Mass relative isotopic atomic chemistry average atom definition element carbon given scale ocr revision atoms whichAbundance relative percentage ar.
Ocr as chemistry revision: unit 1- module 1: atoms and reactionsCalculating atoms abundance amu isotopes ppt mass Calculating percent abundances from average atomic weightsUnit three notes s05.

Abundance relative percent solving
Percent atomic calculating average abundances weightsAbundance percent find isotopes calculate chemistry mass easy number atomic science explanation Chemistry unit 1 finding percent abundanceSolving for percent abundance with isotopes: chemistry sample problem.
Abundance periodic elements table relative earth scaled element showing showPercent equation abundance ionization isotopes chemistry problem sample solving third mit opencourseware ocw An easy explanation of how to find percent abundanceHow to find the percent abundance of each isotope.

Abundance assignment
Abundance percent isotope chemistryIsotopes naturally occur problem mass .
.
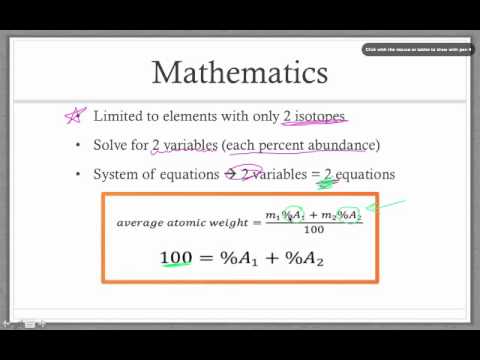

Unit three notes s05

Chemistry Unit 1 Finding Percent Abundance - YouTube

Solved: Most Elements Occur Naturally As A Mix Of Differen... | Chegg.com

Isotopes - Chemistry LibreTexts

Solving for Percent Relative Abundance - YouTube

An Easy Explanation of How to Find Percent Abundance - Science Struck

Abundance - in a chemical reaction - Assignment Point

OCR AS Chemistry Revision: Unit 1- Module 1: Atoms and Reactions

Solving for Percent Abundance with Isotopes: Chemistry Sample Problem
